
Professor Kai Hinrichs is coming from Germany to talk to us about archaea (single celled, microrganisms with no cell nucleus or organelles) on the bottom of the ocean. He describes himself as
'a biogeochemist studying the interactions between microbial life and the carbon cycle on a range of spatial, temporal and molecular scales. I am interested in which and how microbes shape element cycles and what the related environmental consequences are. In order identify and ideally quantify microbial processes, my research group studies the information encoded in distributions and isotopic compositions of organic biomarker molecules in geological and environmental samples, ranging in size from 1 to ~100 C-atoms. My group consists of closely collaborating biologists, chemists, geochemists, and marine geoscientists. In our research projects, we combine analyses of environmental samples with experimental, laboratory-based approaches. Our current research foci encompass the deep subsurface biosphere, methane biogeochemistry, life in extreme environments, development and application of new geochemical-analytical techniques, prokaryotic membrane lipid taxonomy, and the study of paleoenvironments associated with major perturbations of the C-cycle.'
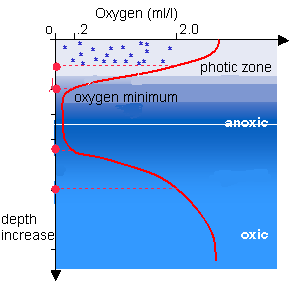
The reading this week is an early nature paper Hinrich wrote discussing the decomposition of a methane hydrate (ice forming in sediment that contains large amounts of methane ), where the methane is being consumed by archaea. As no organism can consume methane without oxygen being present, scientists think that the consumption of methane must be done with a consortium of bacteria with some being sulfate (SO4) reducing (providing the oxygen) and some being a reverse type of methanogen (consuming methane rather than producing it). Hinrichs shows using very negative carbon isotopes that his archaea are feeding on methane and he suggests methane consuming archaea have evolved alongside methane producing archaea. Their presence is important if you want to estimate future or past methane concentrations in the atmosphere (faint sun paradox or at Permian-Triassic Boundary)- because when balancing carbon reservoirs consumption is just as important as production.



 The Permian extinction caused the most fundamental reorganization of ecosystems and animal diversity in the past 500 million years. The marine communities of today are largely a result of the recovery following this extinction. In addition, dinosaurs and mammals arose in the aftermath of the extinction.
The Permian extinction caused the most fundamental reorganization of ecosystems and animal diversity in the past 500 million years. The marine communities of today are largely a result of the recovery following this extinction. In addition, dinosaurs and mammals arose in the aftermath of the extinction.